


neurological and psychiatric illnesses

Clinical research is an essential part of the development of new drugs as well as further developments of known drugs. The development of innovative pharmaceutical forms of known drugs can improvethe safety and/or efficacy in already approved indications. Moreover a known drug can be developed for a new indication. In recent years Desitin has particularly developed galenic optimization of antiepileptic drugs with the goal to improve medical treatment for the patient.
An important example is the development of our sustained release formulations for the treatment of epilepsy: e.g. oxcarbazepine (Apydan® extent, brand in Germany) and valproate (Orfiril® long, brand in Germany). These pharmaceutical innovations demonstrated their improved tolerability and / or compliance if compared in clinical studies in comparison to immediate-release preparations.
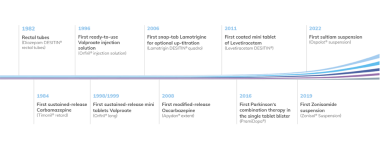
Clinical development at Desitin is mainly focused in diseases of the central nervous system with focus on epilepsy and movement disorders. The clinical development at Desitin comprises primarily pharmacokinetic studies (Phase I) but we also have experience in conducting clinical studies (mainly of phases III and IV) in order to investigate the efficacy and safety of new or known substances in a new indication or in an optimized galenic formulation. Additionally we perform non-interventional studies (NIS) with the purpose to investigate long-term efficacy and/or tolerability of treatment under routine conditions. We cooperate with clinical experts, key opinion leaders and qualified contract research organizations.
An overview of published studies can be find here.